OUR SERVICES
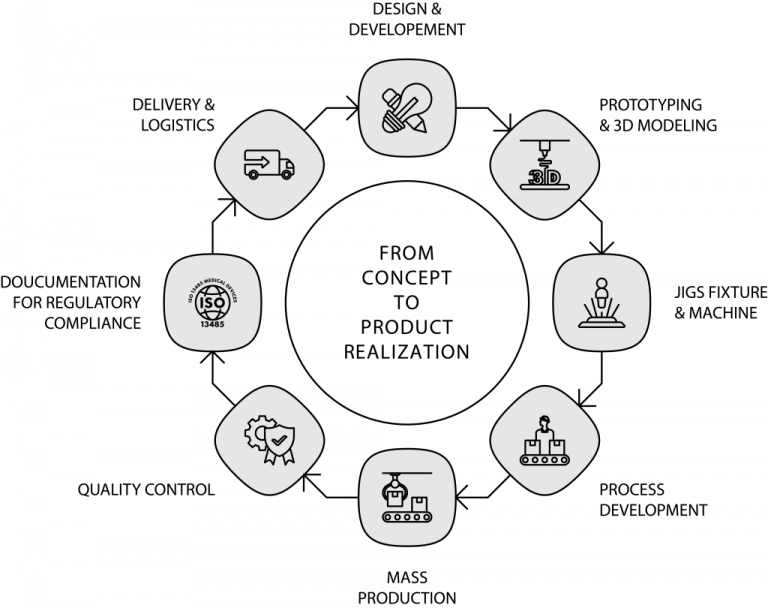
PRODUCT DEVELOPMENT
CCB Medical Devices R&D services are flexible, consistent and proven to manifest your idea into reality. We developed multiple medical device designs with our customers by integrating our comprehensive technical know-how with modern technologies to create products that are robust, safe, cost-effective, environmental and user-friendly.
We provide out-of-the-box and elegant solutions to our customers through our proven multiphase design process to ensure the best products, solutions and shortest development time possible. This proven method captures the entire flow of R&D to ensure value-added collaboration and multifaceted support through frequent communications and proper documentation.
Phase 1 – Design Input
Phase 2 – Design Output
Phase 3 – Review And Design Freeze
Phase 4 – Design Verification And Validation
Phase 5 – Design Transfer
MATERIAL SELECTION
CCB Medical Devices pride ourselves on being limitless when it comes to what we can do with complex materials. Our range of knowledge stretches from the softest polymers to the hard metallic materials, as well as access to Medical grade or FDA certified materials based on your product needs and requirements.
Whether you’re looking to use synthetic or biodegradable polymers, silicone elastomers, engineering plastics, metallic materials, metal replacement alternatives, custom material blend and even if you have no clue to use which materials to use, CCB has the knowledge and access to PhD-level material specialist in-house to assist you in every step.


MATERIAL SELECTION
CCB Medical Devices pride ourselves on being limitless when it comes to what we can do with complex materials. Our range of knowledge stretches from the softest polymers to the hard metallic materials, as well as access to Medical grade or FDA certified materials based on your product needs and requirements.
Whether you’re looking to use synthetic or biodegradable polymers, silicone elastomers, engineering plastics, metallic materials, metal replacement alternatives, custom material blend and even if you have no clue to use which materials to use, CCB has the knowledge and access to PhD-level material specialist in-house to assist you in every step.

TOOLING FABRICATION
With our experienced team of tooling experts, we have handled a multitude complexity of customized tooling development in the aspects of injection mould, blow mould, extrusion pin and die, packaging mould, and other manufacturing tools. We expertly handled multiple pre-existing moulds or developed new moulds using our extensive knowledge across the medical devices industrial
landscape.
Our in-house tooling department and partnered tool-maker are equipped with the latest machines and technologies to ensure all your design needs are taken care of. We provide services such as:
• 3D CAD Modelling & Renderings
• Mould Design for Manufacturing (DFM)
• Mould Flow Analysis
• Design Improvements
• Troubleshooting; and many more
MEDICAL DEVICE TEST VALIDATION & QUALITY SYSTEM
CCB Quality Management System (QMS) was created around the medical requirement and compliant with ISO 13485. The QMS is backed by our experienced Quality Assurance team to meet regulatory requirements through comprehensive and complete documentation. We provide in-house testing services to recommend solutions for quality test plans, test reports, and device protocols.
The effectiveness of our QMS is proven by our extensive track records and a long list of international customers, supporting their compliance to various international medical agencies such as FDA and MDR. The QMS also regularly monitor and collect data through our interconnected back-end system to ensure quality productions while providing customers with data-based solutions and recommendations.


MEDICAL DEVICE TEST VALIDATION & QUALITY SYSTEM
CCB Quality Management System (QMS) was created around the medical requirement and compliant with ISO 13485. The QMS is backed by our experienced Quality Assurance team to meet regulatory requirements through comprehensive and complete documentation. We provide in-house testing services to recommend solutions for quality test plans, test reports, and device protocols.
The effectiveness of our QMS is proven by our extensive track records and a long list of international customers, supporting their compliance to various international medical agencies such as FDA and MDR. The QMS also regularly monitor and collect data through our interconnected back-end system to ensure quality productions while providing customers with data-based solutions and recommendations.
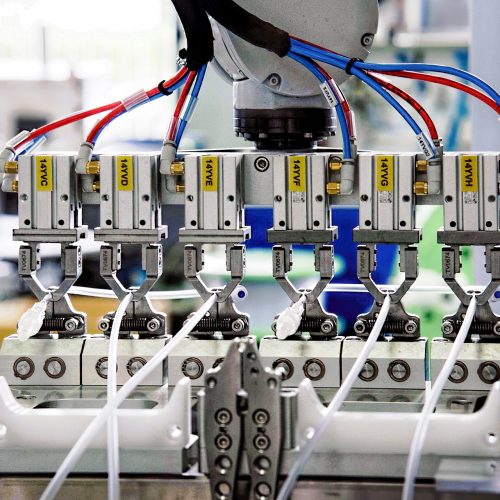
AUTOMATION & JIGS
CCB works closely with customers to enhance the processes for ultimate product quality and consistency. We have extensive experience in optimizing production lines through a right degree of automation solutions such as robots or custom jig fabrication to assist with the intricacy of certain processes.
We strongly believe that each production line should be filled with the minimum required human headcounts to lower the chances of contamination and human error. Automation also leads to better protection and safety for our workers through automatic fail-safe systems. Automation ultimately leads to lower wastage, efficient production and staying true to our philosophy of upholding top product quality.
CUSTOM MEDICAL DEVICE DEVELOPMENT & SOLUTIONS
What if you have a unique product design? Let the experts in CCB work one-on-one with you to realise your bespoke, one-of-a-kind medical devices. Our expert engineers can assist you in developing a prototype to define the product and process design while ensuring its feasibility for mass manufacturing through the combination of CCB’s innovative strength and industrialisation competency. The cumulative 50 years of experience in medical devices allow us to provide not only quality products but efficient and consistent processes, the true hallmark of Product Development.
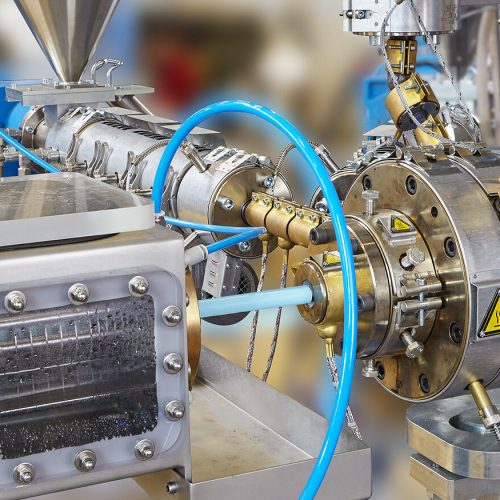

CUSTOM MEDICAL DEVICE DEVELOPMENT & SOLUTIONS
What if you have a unique product design? Let the experts in CCB work one-on-one with you to realise your bespoke, one-of-a-kind medical devices. Our expert engineers can assist you in developing a prototype to define the product and process design while ensuring its feasibility for mass manufacturing through the combination of CCB’s innovative strength and industrialisation competency. The cumulative 50 years of experience in medical devices allow us to provide not only quality products but efficient and consistent processes, the true hallmark of Product Development.
